
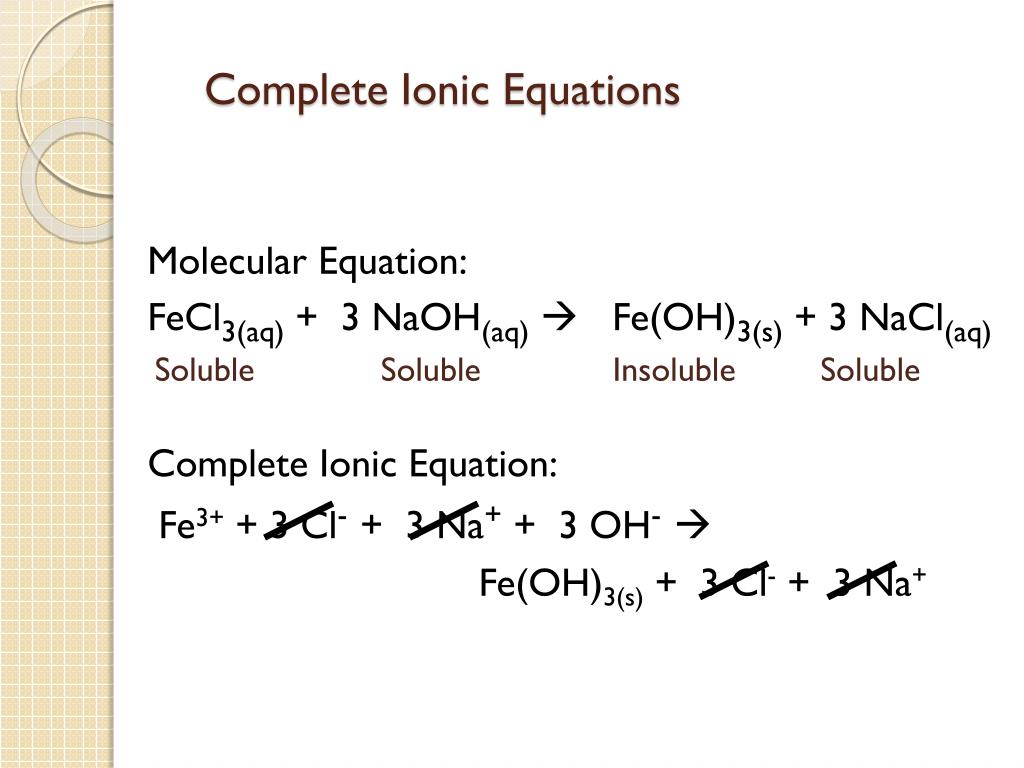
You may notice that in a complete ionic equation, some ions do not change their chemical form they stay exactly the same on the reactant and product sides of the equation. Note: charges in a net ionic equation are conserved. CaCl 2 ( aq) + Pb ( NO 3) 2 ( aq) Ca ( NO 3) 2 ( aq) + PbCl 2 ( s) Answer. Write the remaining substances as the net ionic equation.
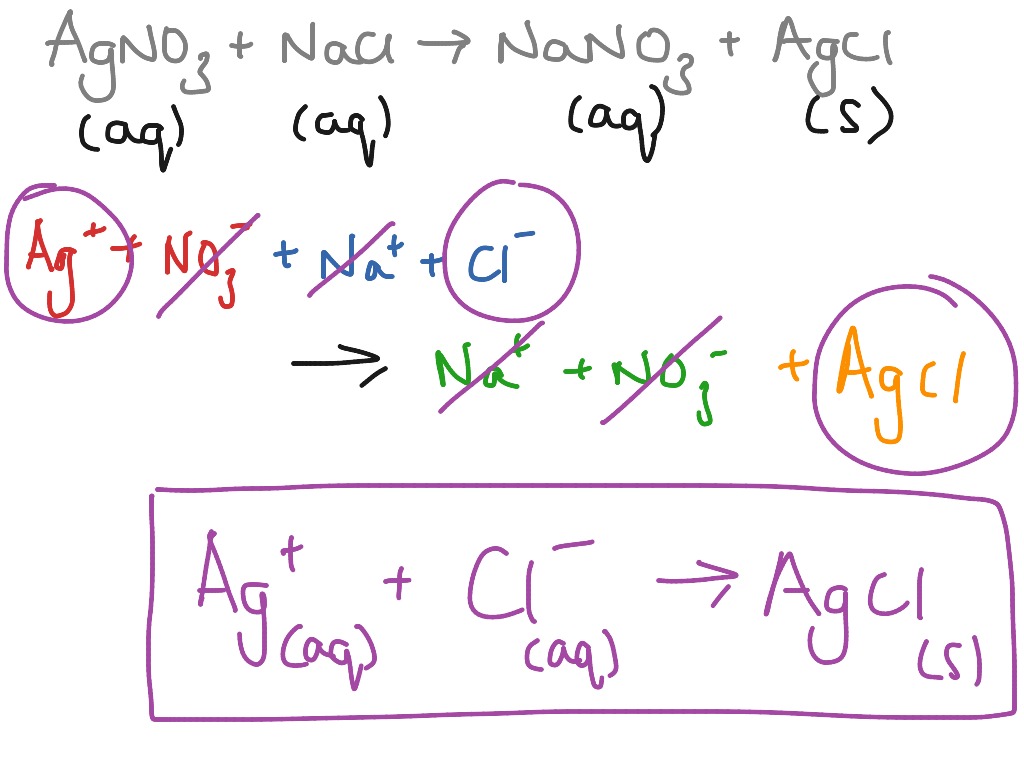

determine if compounds are soluble and will dissociate in water.Humans interact with one another in various and complex. Predict the solubility of common inorganic compounds by using solubility rules. find the charge on ions (both monatomic and polyatomic). Define three common types of chemical reactions (precipitation, acid-base, and oxidation-reduction) Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations.balance molecular equations by changing the coefficients.To be successful, you need to understand how to: Net ionic equations tell us what actually reacted and what we can ignore (called spectator ions). We write net ionic equations to understand what is changing in a chemical reaction. From the balanced molecular equations, write the complete ionic and net ionic equations for the following: K2C2O4 (aq) + Ca (OH)2 (aq) 2KOH (aq) + CaC2O4 (s) a) complete ionic equation b) net ionic equation.


 0 kommentar(er)
0 kommentar(er)
